
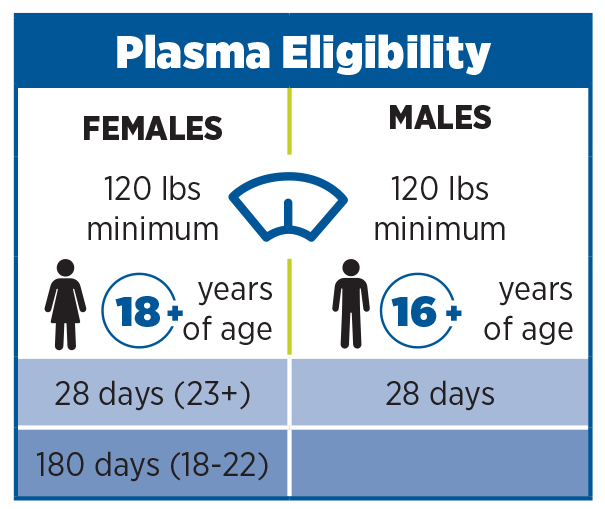
( 1 ) The examination and determination of the donor's health required in § 630.10(f)(2) for donors with blood pressure measurements outside specified limits, or in § 630.15(b)(7) for certain donors who have experienced red blood cell loss However, the responsible physician must not delegate: (A) The activities listed in paragraphs (b)(1)(i) through (iii) and (b)(1)(v) of this section, with respect to Source Plasma and plasma collected by plasmapheresis. (i) The responsible physician may delegate to a physician substitute or other trained person any of the activities described in paragraph (c)(1)(i)(A) of this section, provided that the responsible physician or a physician substitute is on the premises at the collection site: (1) Donor eligibility and blood component collection activities. (c) Which activities related to the collection of Source Plasma and plasma collected by plasmapheresis may the responsible physician delegate ? (2) The responsible physician need not be present at the collection site when activities delegated under paragraph (b)(1) of this section are performed, provided that the responsible physician has delegated oversight of these activities to a trained person who is adequately trained and experienced in the performance of these activities and is also adequately trained and experienced in the recognition of and response to the known adverse responses associated with blood collection procedures.
(v) Other activities provided that the Director, Center for Biologics Evaluation and Research, determines that delegating the activities would present no undue medical risk to the donor or to the transfusion recipient, and authorizes the delegation of such activities. (iv) Obtaining the informed consent of a plateletpheresis donor as described in § 640.21(g) of this chapter or (iii) Returning red blood cells to the donor during apheresis (ii) Collecting blood or blood components The responsible physician may make these determinations by telephonic or other offsite consultation. (C) The determination of the health of the donor and the determination that the blood or blood component collected would present no undue medical risk to the transfusion recipient, as required in § 630.20(c). The responsible physician may make this determination by telephonic or other offsite consultation or (B) The determination of the health of the donor required in §§ 630.10(f)(4), 630.20(a), and 640.21(e)(4) of this chapter. (A) The examination and determination of the donor's health required in § 630.10(f)(2) for donors with blood pressure measurements outside specified limits, or for certain more frequent donations under § 630.15(a)(1)(ii) (i) Determining the eligibility of a donor and documenting assessments related to that determination, except the responsible physician must not delegate: (1) The responsible physician may delegate the following activities to a physician substitute or other trained person: (b) Which activities related to the collection of blood and blood components, other than Source Plasma and plasma collected by plasmapheresis, may the responsible physician delegate ? (a) Who must determine the eligibility of a donor ? The responsible physician must determine the eligibility of a donor of blood or blood components in accordance with this subchapter. Subpart B - Donor Eligibility Requirements REQUIREMENTS FOR BLOOD AND BLOOD COMPONENTS INTENDED FOR TRANSFUSION OR FOR FURTHER MANUFACTURING USE


 0 kommentar(er)
0 kommentar(er)
